DNA PCR Stool Analysis

It is estimated that there are over 200 million cases of Gastrointestinal disease each year in the United States. Acute or chronic diarrhea caused by a bacterial, viral, or parasitic pathogen present in the GI tract, accounts for a significant proportion of the cases. Many of these cases often go undiagnosed or untreated.
The Pathogens Panel measures pathogenic organisms that are known to cause hospital-acquired infections (HAI) (such as C. difficile or norovirus), foodborne illnesses (such as E. coli or Salmonella), and common causes of diarrhea (such as Campylobacter, Shigella, and rotavirus A). This panel measures viral causes of gastroenteritis unavailable by other common stool tests, as well as parasites such as Cryptosporidium, Giardia, and Entamoeba histolytica. Helicobacter pylori and its virulence factors are also included.
For Individuals with Symptoms of:
- Chronic and Acute Gastroenteritis
- IBD-Inflammatory Bowel Disease
- Suspected H. Pylori Infection
- Recreational Water Illness
- Intestinal Inflammation
- Bacterial Infections
- Parasitic Infections
- Viral Infections
Pathogens Panel (stool)
Methodology:
Quantitative Polymerase Chain Reaction (qPCR), is a molecular DNA laboratory technique. PCR amplifies the gene targets and can generate thousands to millions of copies of a single target DNA sequence. This increases the sensitivity and specificity of the test greatly.
PCR, specifically qPCR, can detect slow-growing or difficult-to-culture microorganisms.
Test Ordering Options
- H. pylori with or without Virulence Factors
- Reflex Test Option: Antibiotic Resistance Gene Panel
Test Turn-Around Time
15–18 business days
Specimen Requirements
- Single stool collection shipped ambient.
- Test kits include specimen collection instructions, test request form, collection tray, Carey Blair transport medium, specimen bag, gloves, absorbent material, and a pre-paid Clinical Pak mailer (U.S. and Canada).
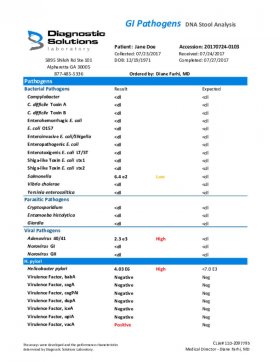
Sample Report